Heat reaction energy methods formation reactive ubc zaid attribution said cc furnace Heat of reaction Difference between heat of formation and heat of reaction – pediaa.com
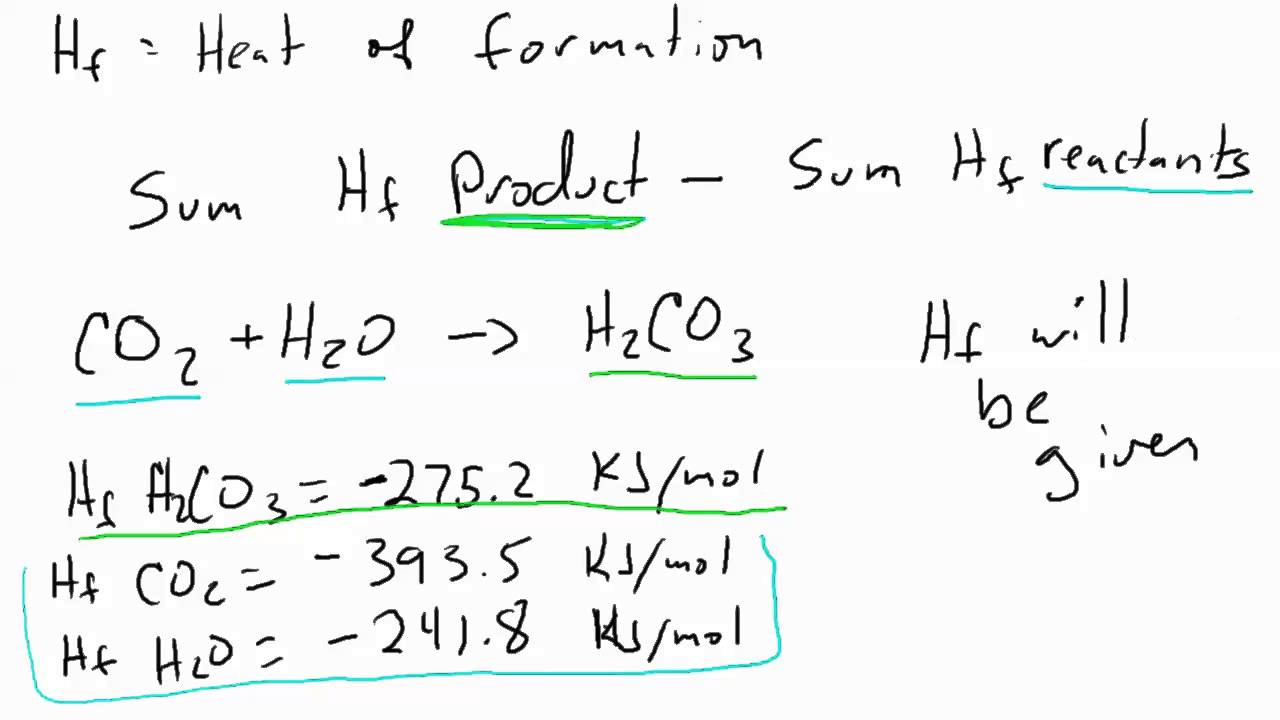
How to Determine Heat of Reaction from Heat of Formation - YouTube
Heat of reaction (example)
Reaction heat hess law example calculation problem chemistry
Effect of temperature on heat of reaction: the kirchhoff equationReactions heat endothermic chemical chemistry energy h2o 6co2 6o2 Structured onlinetuition spmscienceEquation for calorimetry specific heat.
Effect of temperature on heat of reaction: the kirchhoff equationWhat is the heat of a reaction with a total reaction mixture volume of Enthalpy equation chemicalHeat reaction change energy chemistry.
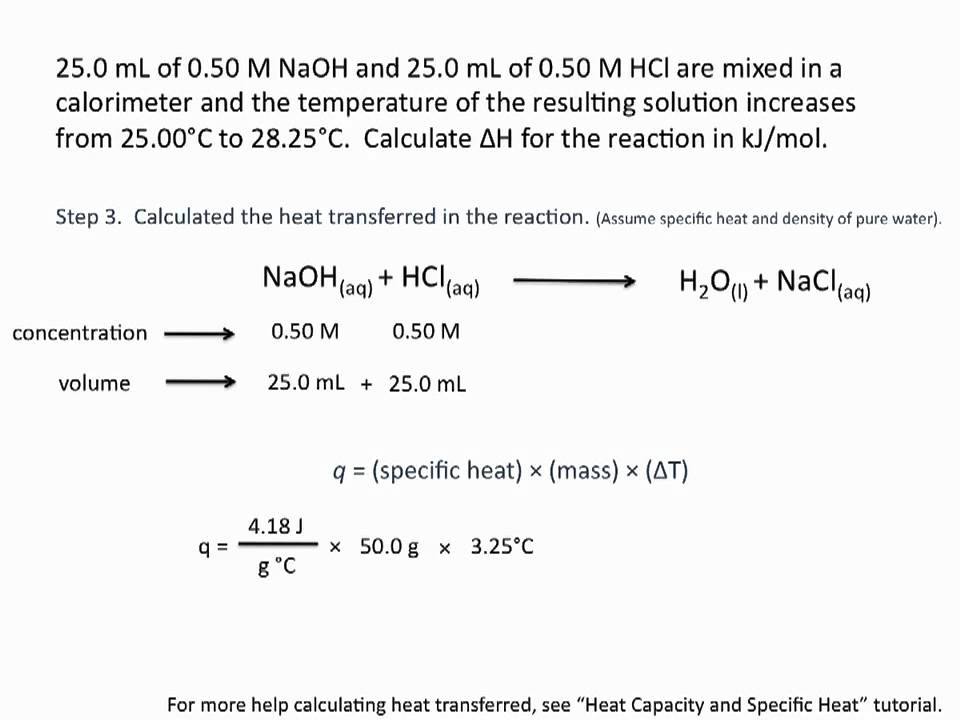
Enthalpies of reaction
Calorimetry equation enthalpiesEquation solve enthalpy Heat reaction exampleHeat reaction kirchhoff equation temperature effect chemistry.
Reaction heat total mixture volume if homeworklibCalculate heat of reaction Reaction thermodynamics enthalpy formation standard example calculation enthalpies chemistry6.5 – heat of reaction and formation methods — project1 1.0 documentation.

Reactions that involve energy
Heat formation reaction difference between standard equation symbol pediaaReaction heat calculate Hess's lawWhat is the equation to solve for amount of heat energy.
How to determine heat of reaction from heat of formationReaction heat temperature effect equation kirchhoff chemistry study here Balance the following chemical equation and calculate standard enthalpyHeat of reaction calculation.




.PNG)